I have read about Saponification when I was in my high school but never gave a thought about it because my mom believed in Ubtan powders right from hair wash and bathing powders. She is a master in it. Till date she enjoys and makes sure she does that in the same way. She is the most loving and care taking mom just like all the beautiful mom out there, who always find time to prioritize their children over their personal growth. So as I kid I have always enjoyed the Ubtan Powders for my skin and hair. Fast forwarding my life to adulthood managing the job, kids and house chores I felt lazy to use the traditional ways. Of course, as a result of my laziness, my glow began to vanish, and I had to forcibly inherit dull and tired skin and hair. I was feeling guilty and wanted to get back to the traditional method. I always wondered how my mom was managing it so efficiently. That gave me way to a new path, combining the good old tradition with a modern twist.
As a consequence of my lack of discipline, my natural radiance faded, leaving me with lackluster, fatigued skin and hair. A sense of remorse washed over me, urging me to revert to age-old practices. I was captivated by my mother’s seamless mastery of these techniques, inspiring me to forge a new path. This involved harmonizing cherished traditions with contemporary innovation.
Cold process soap has been a real remedy for my skin. After countless trial and error batches, I’ve been able to perfect my soap-making skills. I wouldn’t say I’ve reached the end, as there’s always room for improvement and innovation. However, I can confidently say I’ve created some beautiful recipes that have helped rejuvenate my skin. My mom’s approval after using them was the greatest credit and validation for my three years of extensive research and work.
Must Knows before we begin
While churning up a cold process soap is quite easy, a few words of caution are necessary while engaging in this process. The main concern is the handling of chemicals, particularly Sodium Hydroxide (NaOH), which plays a vital role in the saponification process, whether it’s hot process or cold process soap making.
Sodium Hydroxide is an extreme base and is quite corrosive. Therefore never ever forget to use your safety gears like-
- A pair of gloves.
- Mask.
- Safety Goggles.
- Apron.
- Cotton or Denim Clothes.
Do not wear new or good cloths as accidental spill can spoil them. If you have an apron you can use them.
Cotton or Denim clothes will be more comfortable than any other shiny or light weight fabrics.
In case of accidental spill of lye solution do not panic but quickly wash them it excessive amount of water. Other than lye there isn’t any chemicals involved which can cause injuries.
Now, a question might arise: “How can this be called chemical-free?” To answer this, let’s delve into saponification, which will unlock the science behind chemical-free cold process soap.
What is Saponification?
” HOW COULD THIS BE CALLED AS A CHEMICAL FREE PRODUCT?”
To answer this question we need to understand the saponification process.
Saponification is the chemical process of making soap by reacting a fat or oil with a strong alkali, typically Sodium hydroxide (NaOH) or Potassium hydroxide (KOH).
Can you think of any other better base to use in Soap making? If yes, please leave your reply in the comments.
Sodium hydroxide are used in places where you need firm bars. Potassium hydroxide are widely used in liquid soap making.
Coming back to saponification, this reaction breaks down the fat or oil as two main components such as
- Fatty acids, and
- Glycerol
The fatty acids then react with the alkali to form soap.
So what do you think the role of Glycerol in a soap would be? If you know the answer please do comment. If not, keep thinking the short answer follows in the detailed breakdown of the process.
I have a separate post to discuss the benefits of Glycerol aka Glycerin.
Here’s a more detailed breakdown of the process:
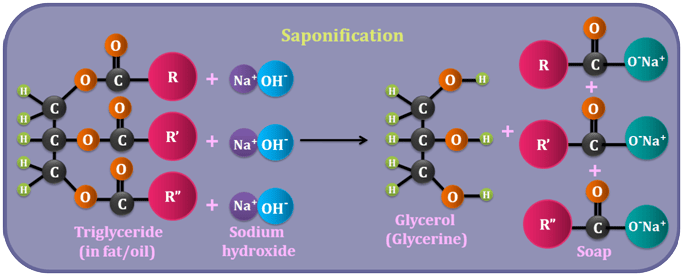
- Starting Materials:
- Saponification uses fats or oils (which are triglycerides) and a strong alkali. Common examples of fats and oils include vegetable oils (like coconut oil) and animal fats. I have written a post on types of oils and butters that can be used in soap making.
- The Reaction:
- The fat or oil is heated with the alkali solution (e.g., sodium hydroxide or potassium hydroxide). This causes the ester bonds in the triglyceride molecules to break.
- Here temperature plays a key role . High or low temperature will drastically affect the soap making process. I have written about the ambient temperature under the steps involved section.
- Products:
- The breakdown of the ester bonds releases glycerol and fatty acids. The fatty acids then react with the alkali to form soap, which is a salt of the fatty acid.
- Soap Formation:
- The soap formed during saponification has a dual nature: a polar “head” that is attracted to water and a nonpolar “tail” that is attracted to oils and fats. This unique structure allows soap to dissolve grease and dirt, making it effective for cleaning.
- Glycerol:
- The other product of saponification, glycerol, is a valuable byproduct used in various applications, including cosmetics and pharmaceuticals.
- Glycerol (also known as glycerin) offers numerous benefits for the skin due to its humectant and emollient properties. It effectively moisturizes, improves skin texture, and protects against irritants, making it a popular ingredient in skincare products.
- Saponification in Soap Making:
- Saponification is the fundamental reaction in soap making, transforming fats and oils into the cleansing agent we know as soap.
How is Cold Process Soap called as Chemical Free?
Now that we read about saponification and its detailed process. We can learn that once alkali is mixed with oils, we get two components-
- Fatty Acids
- Glycerol
So, Where did the Sodium Hydroxide(NaOH) or Potassium Hydroxide(KoH) go?
Well, they got used up to get the above mentioned key components. If proper technique, measurements and procedure is followed. We would get a beautiful cold process soap with no residual lye left behind.
Now that we have discussed about saponification and about the goodness of glycerol. Do we get the same amount of glycerol in the store bought soaps?
We all know the answer. The glycerol that is available in the soap that are whipped at our home either through Hot process or through Cold process has more glycerol content than the store bought ones.
Now I believe you can understand how we can rightfully claim that we are going to indulge ourselves in making CHEMICAL FREE COLD PROCESS SOAP.
Are you ready?

If yes. Get ready to embark on an exciting journey into the world of cold process soapmaking! This marks the very first post in an informative series designed to make this craft incredibly accessible and easy to grasp, no matter your experience level. We’re going to meticulously dissect the entire process, breaking it down into clear, manageable steps. Our goal is to ensure you don’t miss a single detail, empowering you with the knowledge and confidence to create your own unique, personalized soaps that you’ll be proud to use and share!

The creation of beautiful, aromatic soaps becomes a readily attainable goal when the process is methodically broken down into smaller, manageable steps, coupled with the application of careful, and thoughtful attention being given to each stage throughout the entire soap making journey.
Ingredients Required:
A Cold Process Soap making involves only a handful of ingredients like –
- Choice of Oils and Butters
- Herbs (If added)
- Quantity of Sodium Hydroxide
- Chelators
- Boosters
- Additives
- Preservatives
- Perfumes, and
- Suitable Apparatus along with Weighing Scale and Thermometer.

A good quality weighing scale is a must. In fact I use a good kitchen scale and a scale to weigh smaller quantity like 0.01 Gram to 200 Gram Scale. Quite affordable scales are available in amazon.com

Steps Involved:
These ingredients are not such a big deal to source. So, the art of soap making is not that difficult. The real art lies in mixing these ingredients at an appropriate quantity at the appropriate stage with an ambient temperature. Once that step is perfected we are just one stage away from enjoying our bath with our handmade cold process soap and that stage is curing. We can list the steps like this –
- Weighing Oils and Butters
- Weighing Chemicals
- Prepping the Oils.
- Prepping the Chemicals.
- Mixing the Oil and Chemicals at the ambient temperature.
- Mix Mix Mix until trace.
- Add Perfume of your Choice.
- Transfer your soap to Mould
- Wait until the Curing time (45-60 days)
Ambient temperature plays a crucial role in soap making. Sodium Hydroxide when diluted with water to make a Lye Solution usually it is quite hot. It needs some time to cool down and an acceptable temperature would be around 120o F / 49oC. You can get to know more about this in my further posts.
Oils can be infused with herbs and butters need to be melted. So oils and butters should also be around the same temperature as in Lye Solution 120o F / 49oC.
Tah!dah! the soap is all set to be enjoyed. I am so excited to share my experiences in my soap making journey and I love to see and hear people enjoying the same. The prelude for my next post is we are going to start with the first process of weighing oils and butters.